
In the case of gas particles, Entropy is generally higher when compared to solid ones.
#Change in entropy formula free
For example, in case of solid where particles are not free to move frequently, the Entropy is less as compared to gas particles that can be disarranged in a matter of minutes. q res heat exchange for the system for reversible. Entropy is basically a thermodynamics function that is needed to calculate the randomness of a produce or system. Randomness of any system is called entropy of system.
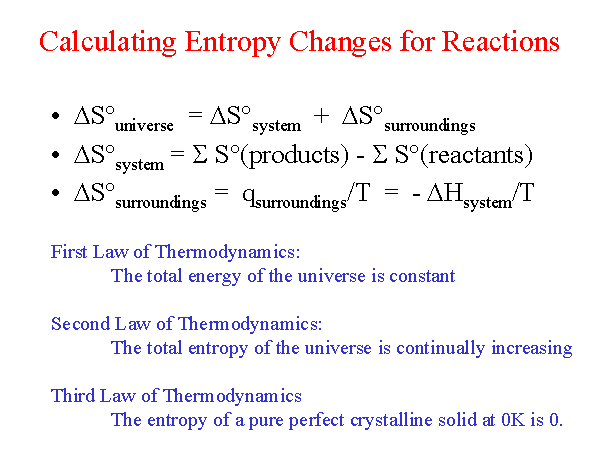
Prob : 5.2 A mass of 5 kg of liquid water is cooled from 100oC to 20oC. Comment : Entropy decreases as heat is removed from the system. Determine the change in the entropy of the body. The heat energy removed in the process is 7875 J. n 1 because you have one mole of gas and R is a constant (8.3145 J/molK). Prob : 5.1 A body at 200oC undergoes an reversible isothermal process. V2/V1 would be 1/3 because you compress the ideal gas to one third of its original volume. This is the formula for change in entropy when temperature is constant. The change in entropy is inversely proportional to the temperature of the system. This was second lay of thermodynamics where the concept of Entropy came into existence. I think you would use the formula deltaS nRln (V2/V1). I hope you must have a better idea of Entropy here with this example. So, from an ordered stage, perfume reached to disordered stage by spreading throughout the room. Another good example to state the definition of Entropy is Spraying perfume at one corner of the room, so what will happen next? The perfume will not stay in one corner only but its fragrance can be felt everywhere.
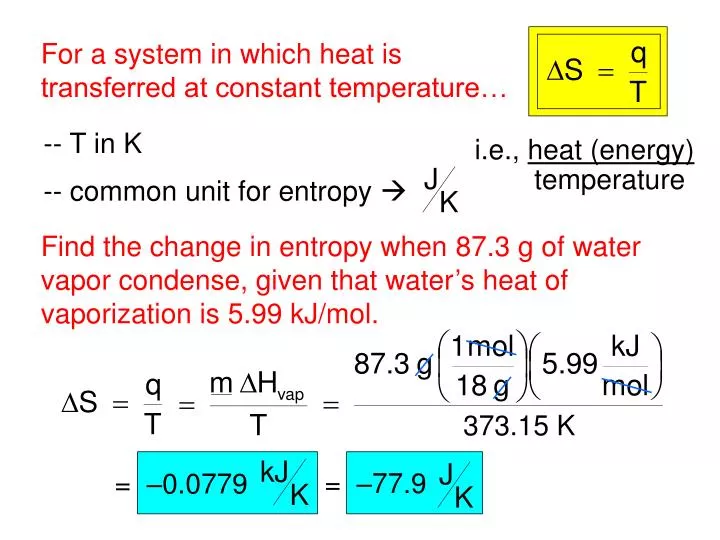
The higher the entropy, the more ways the system is disordered. You calculate the entropy change of a reaction (also known as the entropy change of the system, Ssystem, or just entropy change, S) using the formula S. Let us see how it works actually.Įntropy is defined as the total number of ways how a system can be arranged. Obviously, there are multiple ways to arrange the bag of ball and entropy concept is somewhat similar in Chemistry. The Clausius form of the second law states that spontaneous change for an irreversible process in an isolated system (that is, one that does not exchange heat. Repeat the process until the balls are not ended in the bag. Now draw one more ball from the bag and try to find out the answer of same question. Take an example that you have a bag of balls and if you draw one ball from the bag then how many possible ways to arrange the balls together on the table.


 0 kommentar(er)
0 kommentar(er)
